Scaligerian Datings
The chronology of ancient and mediaeval history in its present form was created and, for the most part, concluded in a series of fundamental works of the XVI-XVII century that begins with the writings of Iosephus Iustus Scaliger (1540-1609), called “the founder of modern chronology as a science” by the modern chronologist E. Bickerman ([72], page 82).

The modern methods of archaeological dating rely on the Scaligerian chronology to a great extent, and may often lead the researcher to great errors, which are blatantly obvious in some cases. Let us give a few examples.
The excavation of a barrow that was “dated with absolute certainty” to the epoch of Kiev Russia (the alleged IX-XII century), according to the “archaeological method,” occurred relatively recently. However, nineteenth-century coins were found in the same barrow, among the bones. This is mentioned in the article by the Belarusian historian Zaikovsky published in History: Fiction or Science? (page 70).
Another example. A different barrow is being excavated, and the archaeologists make another “perfectly certain dating” that ascribes it to the Bronze Age. The ground under the barrow had been virgin until the hole that preceded the barrow had been dug. Some XVIII century ceramics were found in this hole; it could only have got there during the burial. This is yet another case of archaeologists using “scientific methods” for the dating of a XVIII century mound to the Bronze Age, or the time when the rather inexperienced humanity could not have fathomed the intricacies of iron metallurgy. But the XVIII century was a period when both iron and steel were already known quite well. This barrow only got dated to the Bronze Age since it hadn’t contained any steel or iron items.
In the cases described, the barrows contained objects that contradicted their initial datings. If there are no such objects, the archaeologists date the barrows “scientifically” to times immemorial. The very method of “archaeological dating” appears extremely flawed and wholly dependent on the a priori known Scaligerian chronology.
Are Radiocarbon Datings to Be Trusted?
Libby’s Initial Idea. The First Failures
The most popular method claiming the capability of dating ancient artefacts independently is the radiocarbon method. However, the accumulation of radiocarbon datings has exposed the difficulty of the method’s application. According to Oleinikov, “Another problem had to be considered. The intensity of the atmospheric radiation is affected by many cosmic factors. The radioactive carbon isotope production rate should also vary, and one needs to find a method that would take these variations into account. Apart from that, over the period when highways and industrial plants have been introduced by the civilization, a gigantic amount of carbon from the combustion of wood, coal, oil, turf, oil-shales and their products emanated into the atmosphere. How does this atmospheric carbon affect the production of its radioactive isotope? In order to get veracious datings, one has to introduce complex corrections into calculations that reflect the changes in the content of the atmosphere over the last millennium. This issue, as well as a number of technical difficulties, casts a shadow of doubt over the precision of many radiocarbon datings.” ([616], page 103)
Libby in the lab, c. 1960s
W. F. Libby, the author of the method, wasn’t a historian, and did not question the veracity of the Scaligerian datings, which had been used for the justification of his method according to his book. However, the archaeologist Vladimir Milojčić has proved this method to give random errors of 1000-2000 years, while its “independent” dating of the ancient specimens faithfully follows the datings offered by the consensual chronology. Naturally, there can be no talk of “proof” here ([391], pages 94-95).
Let us quote some rather meaningful details. As we have already noted, W. F. Libby had a priori been certain of the veracity of Scaligerian datings. He wrote that they
“…had no contradictions with the historians in what concerned ancient Rome and Egypt. We did not conduct anything in the way of extensive research related to this epoch [sic! – A. F.], since its chronology in general is known to the archaeologists a lot better than whatever our methods could estimate, so the archaeologists were doing us a favour providing specimens [which are actually destroyed, being burned in the radiocarbon measurement process – A. F.]” ([478], page 24).
This confession of Libby’s tells us a lot, since the deficiencies of Scaligerian chronology directly concern the regions and epochs that he and his team “did not research extensively enough.” We can see that the Scaligerite archaeologists were most reluctant about letting the radiocarbon method enter the “certainty epochs” of Scaligerian history for fear of embarrassing discoveries. Archaeologists have naturally got no objections against applying this method to the undocumented prehistory since nothing capable of compromising consensual chronology can possibly be found there.
In what concerns the several reference measurements that were conducted on ancient artefacts, the situation is as follows. The radiocarbon dating of the Egyptian collection of J. H. Breasted “suddenly discovered the third object that we analyzed to have been contemporary,” according to Libby. “It was one of the findings… considered… to belong to the V dynasty [2563-2423 B.C., or roughly four millennia before our time. – A. F.]. It has proved a heavy blow indeed” ([478], page 24).
Why could it have been such a blow? The physicists appear to have restored the veracious dating of the Egyptian specimen, proving the old one to have been wrong. What’s the problem with that? The problem is of course the simple fact that any such dating would prove a menace to the Scaligerian chronology. Carrying on in that vein would lead Libby to compromising the entire history of ancient Egypt. The specimen that Libby had been careless enough to have claimed as modern had to be called a forgery and disposed of ([478], page 24), which is only natural since the archaeologists could not have possibly let the heretical thought of the XVI-XVII century A.D. (considering the method’s precision) origin of the “ancient” Egyptian finding enter their minds.
“The evidence that they [the proponents of the method – A. F.] use for proving the veracity of their method is rather insubstantial, with all the indications being indirect, the calculations imprecise, and the interpretation ambiguous, the main argument being the radiocarbon datings of the specimens whose age is known for certain used for reference… Every time referential measurements are mentioned, everybody quotes the results of the first referential datings that were obtained for a very limited number of specimens [sic! – A. F.]” ([391], page 104).
Libby recognizes the absence of substantial referential statistics. Together with the millenarian dating deviations mentioned above (explained as a consequence of a series of forgeries), we may thus question the very validity of the method as used for dating specimens belonging to the period that we’re interested in, covering the two millennia preceding our century. This discussion does not concern the applicability of the method for geological purposes, however, where millenarian deviations are considered insubstantial.
W. F. Libby writes that,
“there was no deficiency in materials belonging to the epoch preceding ours by 3700 years for checking the precision and the dependability of the method” ([478], pages 24-25).
However, there is nothing here to compare radiocarbon datings to, since there are no dated written documents dating from those epochs. Libby also informs us that his historian acquaintances “are perfectly certain of the veracity of the datings referring to the last 3750 years, however, their certainty does not spread as far as the events that precede this era” ([478], pages 24-25).
In other words, the radiocarbon method has been used most extensively for the period of time that doesn’t allow the verification of the results by any other independent method, which makes life a lot easier for the historians. The example that we quote below is most typical.
“The radiocarbon datings of the three inscription-bearing plaques found in Romania have put archaeologists in a quandary… The ashes that they were found in prove them to be 6000 years old at the very least. Could the discovery of literacy have happened in a rural community in Europe and not in the urban and highly-developed Sumerian civilization? [Such an awful lot of space for the flight of exalted fantasy – A. F.] The scientists consider this probability to be very low… There have been many theories put forward for the explanation of this discovery that apparently refuted the reigning opinion on the origins of written language. Some of the archaeologists, without doubting the scientific principles of the radiocarbon method have suggested the method to be error-prone due to the effects of factors that haven’t been studied as of yet” ([478], page 29).
Could it be that the errors of the method are rather insubstantial and allow for an approximate dating of the specimens belonging to the last two or three millennia? The state of affairs appears to be a graver one. The errors of radiocarbon dating are too great and too chaotic. They can amount to several millennia in what concerns contemporary and mediaeval objects (q.v. below).
In 1984 the Technology and Science magazine had published the results of the radiocarbon method-related discussions from the two symposiums in Edinburgh and Stockholm (No 3, page 9): “Hundreds [sic!] of analysis examples were quoted with dating errors ranging from 600 to 1800 years. In Stockholm the scientists lamented the fact that the radiocarbon method appears to produce the greatest distortions when applied to the history of ancient Egypt in the epoch preceding ours by 4000 years. There are other examples, some of them pertaining to the history of Balkan civilizations… Specialists have reached solidarity in their opinion that the radiocarbon method remains ambiguous due to the impossibility of proper calibration, which renders it unacceptable since it gives no calendar datings.”
A Criticism of the Application of the Radiocarbon Method to Historical Specimens
According to L. S. Klein, the radiocarbon datings “…have confused the archaeologists greatly. Some of them were characteristically overzealous… to follow the advice of the physicists… These archaeologists hastened to reconstruct the chronological schemes [which implies they aren’t constructed firmly enough – A. F.]… The first archaeologist to have opposed the radiocarbon method was Vladimir Milojčić, who… attacked the practical usage of radiocarbon datings, and… criticised the very theoretical foundation of the physical method sharply and bitterly… The comparison of individual measurements of modern specimens with their average value allowed Milojčić to support his scepticism with a series of brilliant paradoxes.
The shell of a living American mollusc has the radioactivity index of 13.8 as compared to the average value of 15.3, which makes it 1200 years old. A live North African wild rose flower with the radioactivity of 14.7 has been dead for 360 years, according to the physicists… as for the Australian eucalyptus with a radioactivity of 16.31, it isn’t likely to exist anywhere in the next 600 years. A shell from Florida with a value of 17.4 shall only appear in 1080 years… Since in the past radioactivity wasn’t distributed any more evenly than it is now, similar fluctuations and errors may afflict ancient objects as well. A prime example is the result of the radiocarbon dating of a mediaeval altar fragment from Heidelberg… which demonstrates that the wood used for the repair of the altar hadn’t existed at that time… In the Iranian Welt cavern the lowest layers were dated to 6054 B.C. (give or take 415 years) and 6595 (give or take 500 years) before Christ, whilst the layer on top was dated to 8610 B.C., give or take 610 years. The upper layer is thus 2556 years older than the lower, which is clearly an impossibility. There is a vast number of similar examples…” ([391], pages 94-95)
Thus, the radiocarbon dating method can only be used for the approximate datings of objects whose age amounts to dozens of millennia, when the error rate is comparable with the actual specimen age reaching one-two or more thousand years. Live molluscs have been dated with the radiocarbon method, and proved to be 2300 years old as a result, which is perfectly preposterous (q.v. in Science magazine, No. 130, dated 11 December 1959). The radiocarbon dating deviation amounts to twenty-three hundred years here.
A few more examples of relatively recent radiocarbon datings made around 1970-1971:
- No. 225 of Nature magazine dated 7 March, 1970 reports the results of analyzing the C-14 content of organic material contained in the mortar of an English castle which is known to have been built 738 years ago. The radiocarbon dating gave the age of 7370 years as a result, being 6500 years off the mark. The radiocarbon dating deviation amounts to six millennia and a half. One wonders whether there was any point in quoting decades with such precision.
- The radiocarbon analysis of seals that have just been shot defined their age as 1300 years, i.e. dating mistake of 1300 years. Seals mummified 30 years ago have been dated as 4600 years old, with a dating error of 4570 years. Quote from the Antarctic Journal of the United States, No. 6, 1971.
The above examples demonstrate that radiocarbon dating can make the specimens thousands of years older than they really are. As we have seen, there are examples of the opposite, when the specimen is dated as belonging to the distant future. One shouldn’t wonder about radiocarbon analysis making mediaeval objects fabulously old.
Let us return to L. S. Klein’s review. He writes that: “Milojčić suggests to cease the tendentious “critical” editing of the radiocarbon datings, which is constantly done by the physicists, and calls upon their patrons the archaeologists to do away with the “critical” censorship that axes the publication of the complete result. He appeals to both physicists and archaeologists to publish all of the results of their research without filtering out the dates that strike them as improbable. He also tries to convince the archaeologists to stop the practice of familiarizing the physicists with the age of the finding, and not giving them any figures until they publish theirs! Otherwise, after such editing, which reflects the private viewpoints of the researchers themselves, the dating is bound to be subjective, so the study of the concurrence between historical and radiocarbon datings becomes impossible.
Thus, in Groningen, where the archaeologist Becker has been a supporter of the short [European – A. F.] chronology, radiocarbon datings are usually recent, whereas in Schleswig and Heidelberg, where Schwabedissen and others have been proponents of the longer version of chronology, these datings are usually a lot more ancient.” ([391], pages 94-95) We think that no commentary to the above is required.
We may be told that the radiocarbon method may have attained a higher level of precision over the last couple of years. This may be true concerning the theory and the actual measurements. The question is, however, whether these improved methods are used in modern archaeological practice, and if so, what results are obtained in this manner. Do the new radiocarbon datings concur with Scaligerian chronology?
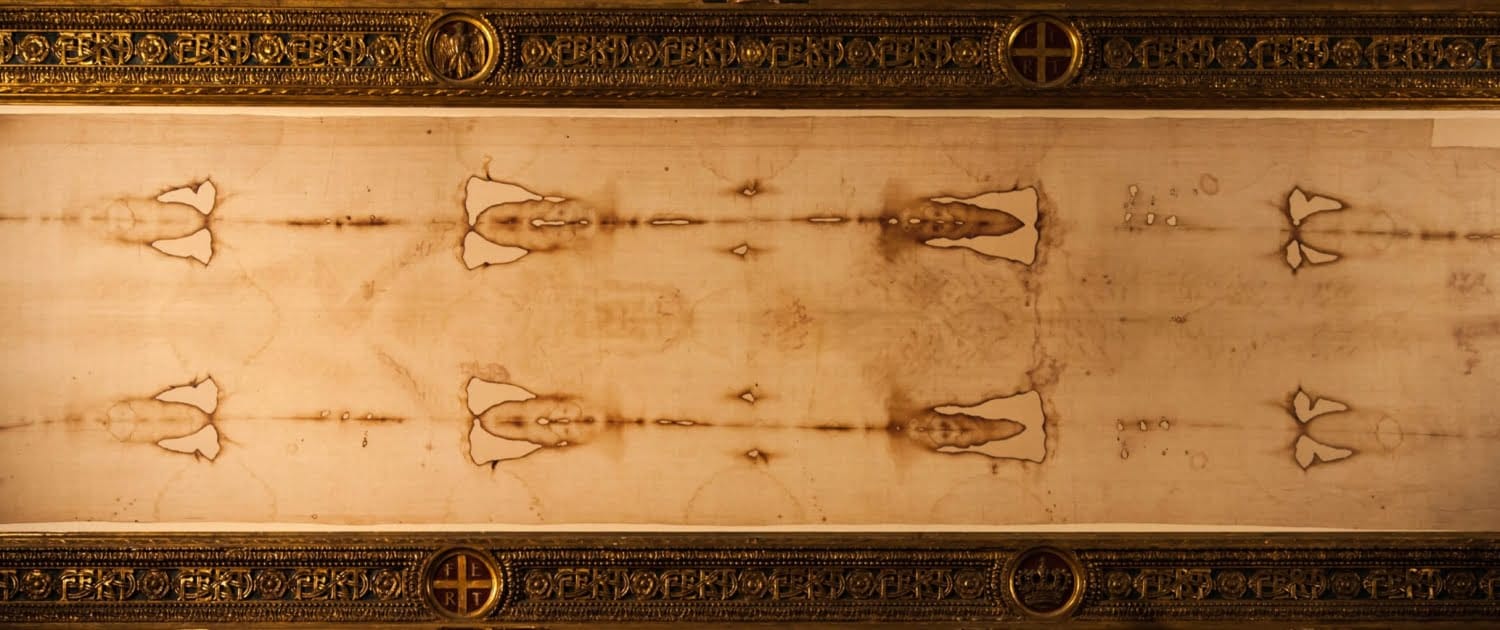
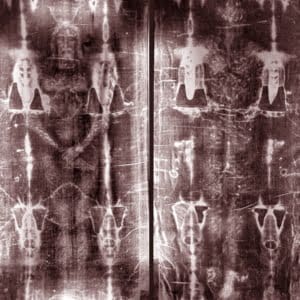
The Dating of the Shroud of Turin
The reports of the radiocarbon dating of one of the most famous Christian holy objects – the Shroud of Turin, q.v. in figs. 1.59, 1.60, 1.61 – caused a great resonance in 1988. According to the traditional version, this piece of cloth bears the image of the body of crucified Christ and dates from the I century A.D., which is supposed to make it about two thousand years old. However, radiocarbon datings have given a different dating: roughly XI-XIII century A.D. The radiocarbon analysis has been conducted in three laboratories – in Oxford University, Arizona University, and the Swiss Technological Institute in Zurich ([769], page 80).A scientific work specifically dedicated to the radiocarbon dating of the Shroud of Turin claims the linen fabric that the shroud is made of to be produced between 1050 and 1350 A.D. ([1055], page 141). The authors cite the results of the Shroud’s radiocarbon analysis performed in the laboratory of the Oxford University ([1055], page 140). The laboratories of Arizona and Zurich have given more recent datings, 1304 and 1274 (with the error rates of 31 and 27 years) respectively ([769], page 82).
These results have proved shocking for many. “In September 1988… a report appeared telling of the analysis and the fact that it gave a certain dating of the shroud’s fabric which turned out a thousand years more recent than the alleged date of Christ’s death… even if the Shroud is dated as a XI century artefact…” ([46], page 25). The author ceases the discussion of the dating after this, and begins to ponder the veracity of Christ’s image as seen on the Shroud.
One arrives at the following conclusions:
- Either the Shroud of Turin is a forgery;
- the radiocarbon datings can contain errors of several centuries or even millennia;
- or the Shroud of Turin is original, but dated to the XI-XIII century A.D. If this be the case, it is natural to ask about the century that Christ’s lifetime pertains to. Could it really have been the XII?
We discuss the radiocarbon dating of the Shroud in our book entitled “King of the Slavs”. The second half of the XII century turns out to be the most likely dating. As we demonstrate in our book entitled “King of the Slavs”, the radiocarbon dating of the Shroud (the middle of the XII century) concurs with other independent datings of Christ’s lifetime. In particular, he must have been born in 1152 and crucified in Constantinople in 1185. We must note right away that our attitude towards the results of radiocarbon datings is highly critical (we shall discuss the reasons at length below). However, the situation with the dating of the Shroud is somewhat different. The specimens of its fabric were dated by a number of different laboratories, which makes the results of this research somewhat more plausible.
The radiocarbon dating of the Shroud of Turin to the XI-XIII century A.D. made the historians rather worried, and provoked a series of attempts to refute the result. A. Agureyev, the ITAR-TASS correspondent, had made a report from New York in 1998 that can be found printed in the Gudok newspaper dated 4 April 1998. This report stated that the radiocarbon dating of the shroud “contradicts the Biblical tradition. However, according to the scientists of the University of Texas, their Italian colleagues should not have used the radiocarbon analysis system”. The Shroud could allegedly “have fallen prey to a fungus” in the XI-XIII century; this may have affected the radiocarbon dating. “However, the scientists have no opportunity of conducting further research, since the Catholic Church refused to provide any more specimens, and even insisted on the return of all of the ones that were at scientists’ disposal” (same source).
Since the results of the radiocarbon dating of the Shroud gave results that contradicted the Scaligerian dating of the life of Jesus Christ, the radiocarbon method had to be exposed to public attention. The protection of the Scaligerian dating of Christ’s life had been provided by the publication of new facts important enough to considerably aggravate the dubiety of the radiocarbon method in what concerns its applicability to historical chronology, already great enough. Let us quote some of the critical materials belonging to the proponents of the Scaligerian chronology ([358]). The publication belongs to Rev. Gleb Kaleda, a prominent geologist, Professor, and Doctor of Sciences. Also see [717] for critical material.
“There are several other factors, either local or planetary, that affect the concentration of C-14 in the atmosphere, hydrosphere, and organic matter, thus complicating and limiting the use of the radiocarbon method in chronology.
- Natural or artificial radiation. Neutrons released in nuclear and thermonuclear reactions, as well as cosmic rays, turn N-14 into C-14. The atmosphere content of C-14 had doubled in the period between 1956 and August 1963. A drastic increase in C-14 content began after the thermonuclear explosions in 1962.
- The local effect of volcanic gases on C-14 content had been described by L. D. Sulerzhitsky and V. V. Cherdantsev ([717]).
In a number of cases radiochronological age calculations give results that are clearly absurd and contradict the entirety of accumulated geological and palaeontological data. In such cases “absolute chronological figures” are to be ignored as blatantly erroneous. The discrepancies between geochronological definitions using different isotope methods may reach a factor of 10x.
In 1989 the British Science and Technology Council analysed the precision of the radiocarbon method (see the 8th issue of the New Scientists magazine for 1989). 38 laboratories from all across the world were involved in the research. All of them received specimens of wood, turf, and carbonate salts whose age had only been known to the organizers of the experiment, and not to actual analysts. Only seven laboratories (of thirty-eight! – A. F.) reported satisfactory results; others proved wrong by factors of 2x, 3x and higher. The comparison of the data received by different researchers that used various analysis methods has shown that the causes of the dating errors were not limited to the imprecision of a specimen’s radioactivity estimation as it had been assumed; apparently, the technology of preparing specimens for analysis had also served as an entropy agent. The diagnostic errata are caused by the calefaction of specimens as well as some methods of preliminary chemical processing. Everything points at the necessity of using the radiocarbon dating method with the utmost caution” ([358], pages 14-16).
In 1997 the German authors Christian Blöss and Hans-Ulrich Niemitz have published a book titled suggestively enough C-14 Crash ([1038]). They have collected a great body of modern material demonstrating rather convincingly the fact that the radiocarbon method in its current form cannot serve as a valid basis for absolute datings of historical artefacts. More on the subject can be seen in the bulletin [1491] that contains the following critical publications of 1991-1995 that interest us:
- Christian Blöss and Hans-Ulrich Niemitz (1996), Der Selbstbetrug von C14-Methode und Dendrochronologie;
- Hans-Ulrich Niemitz (1995), Die “magic dates” und “secret procedures” der Dendrochronologie;
- Herbert Illig (1991), Dendrochronologische Zirkelschüsse.
As we can see, radiocarbon dating might prove more or less effective in analyzing objects whose age is measured by tens and hundreds of millennia. The errors of tens and thousands of years naturally inherent in the methods are of minor importance here, although this is far from obvious. However, the mechanical use of the method for the dating of objects no older than two thousand years, which is the historical epoch that interests us most in what concerns the reconstruction of the true history of documented civilization, appears perfectly impossible without being preceded by extensive and detailed statistical research and calibrations employing specimens of known ages. As far as we know, no such research has ever taken place, so there are no referential statistics. There is also no knowledge of whether improving the method’s precision is a possibility at all. Also see [718].
Other physical dating methods do exist; unfortunately, the spectrum of their applicability is considerably more limited than that of the radiocarbon method, and their precision is also insufficient for the historical epochs relevant to our ends. For instance, in the early XX century some scientists proposed to define the ages of buildings by the shrinkage of their foundations or the deformation of columns; however, no steps have been made in this direction due to the impossibility of calibrating this method and estimating the real shrinkage and deformation speed.
Two more methods have been suggested for dating ceramics: the archaeomagnetic method and the thermoluminescent method. However, they have calibration issues of their own. The archaeological datings offered by these methods for Eastern Europe, for instance, are limited to the Middle Ages.
Let us return to the Shroud of Turin for a second in order to put forth the following hypothesis concerning the nature of the alleged human figure that one sees on the Shroud’s fabric. One shouldn’t exclude the possibility that an embalmed body had really been wrapped in this linen at some point. Let us recollect that the “ancient” Egyptians had the practice of wrapping a body up in several tight layers of cloth saturated with various elixirs. This may have resulted in a “carbon copy” of a body on the fabric of the cloth which was later removed for some reason, and stored with great care. See our book entitled “King of the Slavs” for more details.
Summary
Let us sum up the information that we have just considered. We have learnt that the real activity of ancient specimens may alter from the average value for the following reasons:
- A temporal change in timber activity: 2% deviation range;
- Cosmic ray intensity changes (theoretical estimation): 20% deviation range;
- Short-term changes of solar activity: additional 2%;
- An increase in the mixing rate of the oceanic water: minus 2%;
- Variations in radiocarbon concentration depending on the geographical location and the tree species: 8.5% deviation range;
- Variations in radiocarbon content resulting from decomposition processes: ? (unknown);
- Variations in radiocarbon content resulting from a specimen’s chemical processing: ? (unknown);
- The variations in the exchange reservoir radiocarbon content resulting from the washing out of carbonate rock formations: ? (unknown);
- Variations in radiocarbon content caused by large quantities of carbonates produced by volcanic eruptions: ? (unknown). This reason can provide for significant distortion of radiocarbon datings for the areas close to volcanoes, such as Italy with its Vesuvius and Etna.
One should also bear in mind the dating deviation resulting from the temporal gap between the cutting of a tree, for instance, and the use of the wood for the object or building researched. Finally, one has to consider the imprecision of the currently used C14 half-life value, that has been corrected by almost 10% as of late, and the errors of experimental measurement of a specimen’s radioactivity (background radioactivity consideration etc). We do not cover these errors (whose correction has cost the physicists lots of labour) presently, since having learned of all the factors mentioned, we deem it nonsensical to attempt the precise measurement of a value whose theoretical uncontrolled error rate may equal 10% if we’re to make modest assumptions. The most optimistic calculations give a radiocarbon dating uncontrolled error range of 1200 years of arbitrarily added or subtracted age.
This makes the placidity of the following conclusion made by B. A. Kolchin and Y. A. Sher most peculiar indeed: “Summing up the brief overview of the centurial C14 variation research, one has to point out that apart from its mere failing to undermine the trust that we have in radiocarbon chronology, this research had made its precision even higher (?! – A. F.)” ([414], page 8). Another specialist in radiocarbon datings, S. V. Boutomo, is of a more realistic opinion: “due to the considerable fluctuations of C14’s specific activity rate, the radiocarbon datings of relatively young specimens (under 2000 years of age) cannot be used as fundamental referential data for the absolute chronological scale” ([110], page 29). However, from the point of view of the “Classical age” studies, including those of the “ancient” history of Egypt, these “relatively young specimens” are of the greatest interest. Thus, certain specialists in the field of radiocarbon dating confess openly (albeit in special scientific literature) that the use of the radiocarbon method in its current state for the specimens whose age is 2000 years or less appears a most dubious endeavour.
We could have finished our overview of the radiocarbon dating method here if it hadn’t been for the criticisms of the method coming from archaeologists and certain oddities in the behaviour of the radiocarbon method specialists themselves. We have quoted some of the examples above. The first thing to attract one’s attention is the absolute trust of the authors in the infallibility of historical datings, as one sees from such passages as “the ages of specimens younger than 5000 years concur well (?! – A. F.) with the historical estimations” ([986], page 155). Such statements appear very odd indeed considering what we have just learnt.
Libby wrote that “further research has been undertaken involving specimens of known ages… The results… span a historical period of 5000 years… Thus, the general reliability of the radiocarbon method is well-proven” ([986], page 135). As we have already demonstrated, the popular myth of the “concurrence” between the Scaligerian chronology and the radiocarbon datings is based on flimsy foundations, and proves immaterial at closer study; the myth’s popularity is clearly of an unnatural origin. Let us remind the reader of something that Libby himself has mentioned in this respect: “One of the exceptions was discovered when we have worked on the materials of a large collection collected by James H. Breasted in Egypt together with the specialists of the well-known Chicago Institute for Oriental Studies. The third object suddenly turned out to have proved modern after analyzing. The finding belonged to a collection ascribed to the time of the V dynasty. It had really been a heavy blow” ([478], page 24). As we have already mentioned, this object was claimed a forgery. The fact that Libby mentions this “strange occurrence” makes one wonder how many of those he remained taciturn about.
As we have already demonstrated, the calibration of the radiocarbon method has been largely based on the Scaligerian chronology. It would be expedient to check whether the radiocarbon method can actually be made independent from written sources. Libby cites the table of modern carbon activity for various rock formations claiming that “it has been shown that there are no significant differences between the studied specimens collected at various latitudes from pole to pole” ([480], page 191).
Wait a second, we have just learnt that the deviation range equals 8.5% in one direction or the other, that is, over 700 years. How is it possible to claim five pages further on that “the carbon content that we have estimated concurs well with the expected value, all deviations being nothing but acceptable reference point errors” ([480], page 196). Could it be that Libby had been certain that the readers would not be interested in the details of Anderson’s table? Libby also says that their “conclusions may have proved wrong if the measurement errors of all kinds – those of cosmic ray intensity, mixing rate and ocean depths, had been in correlation. However, since this is not the case, we reckon that large error rates are improbable” ([480], page 193).
We are not quite certain as to what kind of improbability is being talked about here, since the cosmic ray intensiveness, mixing speed, and other physical values affecting the initial radiocarbon content in a specimen for the moment of its departure from the exchange reservoir are far from being random – all of these values had all equalled something at a given point in time. If we do not know these values and have to make a choice from some interval of possible values, the radiocarbon dating error shall equal the sum (!) of all the errors that have been made in the estimation of the source data for the specimen.
Libby writes that “despite the great differences between the cosmic ray intensiveness values at different geographical latitudes (they are a lot higher in the northern and southern latitudes than they are around the equator), one has to expect (? – A. F.) the radioactive carbon propagation rate to be homogeneous for the entire planet” ([478], page 23). The effect mentioned may nevertheless result in “extra age” gathered by specimens in Egypt, for example.
Libby proceeds to tell us the following: “The coincidence of the age of the core and the entire tree shows that the sap from the core of gigantic sequoias is not chemically balanced in comparison to the fibre and other molecules of the tree. In other words, the carbon in the central part of the tree had been stored there about 3000 years ago, although the actual tree had only been cut down several decades ago” ([480], page 195).
However, three years after this, the radioactivity of tree rings was researched by Süss, who has found the discrepancies between the radiocarbon datings and the dendrochronological ones. Did he make the conclusion that Libby’s initial hypothesis was wrong? He did not. Süss made the claim that the radiocarbon content in the ancient times used to be higher than it is today instead. What we see is a vicious circle.
L. S. Klein gives a similar example in [391]. First Libby proves the veracity of the radiocarbon method using the historical chronology of the “ancient” Egypt; however, when control measurements showed deviations, Libby immediately questioned the Egyptian chronology concerning these particular specimens ([391], page 104). Similarly, Libby had used dendrochronology in support of the radiocarbon method, explaining arising deviations by the fact that several tree-rings may be formed in a year. However, Libby is far from being the only one to demonstrate the lack of logic where its presence is undesired.
In the article by Kolchin and Sher ([414]) we read that “the dates calculated in assumption of the constancy of atmospheric C14 content from the ancient times to our age need to be revised. Does this mean they aren’t true? The following analogy appears congruent…” ([414], page 6). The authors proceed to tell us how the distance between the Earth and the Moon had been calculated in several stages, each time with a greater precision. The same allegedly applies to the radiocarbon method where gradual corrections make the calculations more precise as time goes by. This may well be so in theory. However, we read in the very same article that “the half-life period for C14 is 5570 years, with the possible deviation range of 30 years in each direction…” (page 4), and that “the half-life period for C14 is set (!? – A. F.) at 5730 years, give or take 40”. 160 years – that’s some correction!
M. J. Aitken writes that “an important characteristic of all these methods is their output, that is, the carbon content in the original volume that is transformed into gas. It would be expedient to have an output of 100% in order to eliminate all possibility of C14 turning into gas more readily than C12, or the other way round” ([986], page 168). We also learn that “the shortcoming of the synthesis of the latter is that only 10% of the carbon is transformed into benzol; this increases the possibility of error resulting from isotope separation” ([986], page 17). The author appears to have full awareness of the necessity of considering the isotope separation effect in all chemical reactions. However, in 6.3, while discussing the issues of a specimen’s suitability for measurements, M. J. Aitken writes that “charcoal and wood in good condition are considered the best specimens: their taking part in exchange is improbable (? – A. F.), and the only possible kind of decomposition results from the production of carbon oxide or dioxide. However, this process isn’t relevant to us, since it only concerns the carbon lost by an object” ([986], page 149). What about isotope separation? The radiocarbon content in a specimen may change as a result of decomposition!
Such careless attitude of specialists to the effects that may greatly affect the research results remains enigmatic for us. We have listed some of these effects in the general list. Some of them may really be difficult to evaluate currently. However, a number of effects reflected in literature may be quantitatively assessed after a series of experiments. No careful activity reports of either living or dead specimens have been made for any of the below:
- latitude;
- longitude;
- proximity to certain geological and geographical formation on dry land and in the ocean;
- altitude above the sea level;
- climate etc.
Without such analysis, the self-righteous claims of the alleged independence of specimen activity from their locations and other characteristics are altogether impossible to understand.
Therefore, we have to concede the following:
- The radiocarbon method in its current condition has deviation rate of 1000-2000 years for specimens whose age is estimated as under 1000 years. This means there’s not much to be learnt about the events of the last two millennia from this method.
- The radiocarbon method needs a fresh graduation that would not be based on Scaligerian chronology at the very least.
- Other physical dating methods are even less precise, ergo, they can tell us nothing about the dating of objects younger than 2000 years.
- The actual archaeological methods that aren’t based on documented chronology can give no absolute dates; these methods can only aid the estimation of relative chronology of some findings in a limited number of cases.
- Scaligerian chronology implicitly or explicitly affected the graduations of scales used for archaeological and even physical methods, including the radiocarbon method. This also questions the usability of the method in its current condition for the dating of historical objects.
- According to a number of archaeologists (see above), the unacceptable practice of familiarizing the physical laboratories that perform radiocarbon datings with the opinions of the archaeologists about the estimated ages of findings still exists.